Innovative, Intelligent AMT Powered by Ophthalogix
Better for your patients, better for your practice. Aurora heals eyes, naturally.
Embrace the future of eye care with Aurora, where medical innovation leads to better patient outcomes, naturally.
Aurora’s groundbreaking feature is a strategically placed hole, or aperture, in the center of the tissue.
The aperture spares patients the reduced vision post-procedure associated with first-generation grafts, permitting maximal oxygen flow to the central cornea, supporting corneal well-being and eliminating the risk of central hypoxic keratitis and its sequelae.
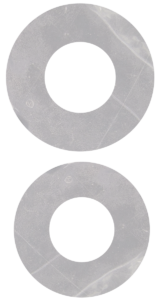
AURORA SIZES
12 mm with 5mm aperture
- EAS-90012 – Aurora Single Layer 12mm
- EAD-90012 – Aurora Dual Layer 12mm
10 mm with 5mm aperture
- EAS-90010 – Aurora Single Layer 10mm
- EAD-90010 – Aurora Dual Layer 10mm
FORMULA/5™ Processing Technique
The FORMULA/5™ processing method is designed to retain natural growth factors and other regulatory proteins to support the full healing cascade. 1,2,3,4,5
- Ensures minimal manipulation
- Uses no harsh chemicals
- Provides the most gentle drying process in the industry
- Engineered to meet stringent FDA regulations and AATB standards
- Room-Temperature Storage
- Quick Rehydration
- Easy Application
1. Partial list of regulatory proteins. 2. Data on File. Ophthalogix can provide information upon request from C5 Biomedical, manufacturer of Aurora. 3. Koizumi N, et al. Current Eye Research. 2000; 20(3): 173-77. 4. Koob TJ, et al. J Biomed Mater Res Part B 2015; 103B: 1133–40. 5. Li JF, et al. HGF accelerates wound healing by promoting the dedifferentiation of epidermal cells through 1-integrin/ILK pathway. Biomed Res Int. 2013;2013:470418. doi: 10.1155/2013/470418. Epub 2014 Jan 15. PMID: 24490163; PMCID: PMC3899705.
Safe, effective and natural, Aurora AMT is an innovative, patent-pending, minimally manipulated tissue designed to greatly reduce visual obstruction that often accompanies an amnion membrane placement.
Unlike other AMT grafts, Aurora features a strategically placed aperture in the center of the tissue, allowing patients to regain their vision immediately after the procedure.
Better for your patients, better for your practice. Aurora heals eyes, naturally.
AURORA BENEFITS
MINIMAL VISUAL DISRUPTION
Unlike conventional amnion membrane grafts, Aurora’s unique aperture enables patients to return to vision immediately after application, allowing no disruptions in daily life or routine.
ENHANCED COMFORT
Aurora is designed for patient comfort, ensuring minimal vision disruption after application.
VERSATILE APPLICATIONS
Aurora can treat a wide range of eye conditions, including corneal defects, conjunctival disorders, burns, scratches and more.
NATURAL HEALING PROPERTIES
Aurora AMT is derived from full-term, pregnant, consenting mothers who c hoose to donate their birth tissue for the greater good. The birth tissue has inherent healing capabilities and can promote rapid tissue regeneration.

Aurora has the following advantages:
- Aurora is air-dried not freeze-dried
- Aurora can be kept at room temperature, eliminating the need for freezer
- Suitable for immediate use off the shelf
- Hydrates rapidly
- Adheres to the ocular surface
- Sterilized with gamma radiation to SAL 10-6 in accordance with ISO 11137
- Textured surface facilitates ease of handling and placement
- Symmetric graft allows either side to be placed in contact with ocular surface
- Can be placed in either direction (multi-directional graft)
- Has a micron thickness of 30-35 Microns
- Tackier stromal surface for better adherence
- Only 9 units of KiloGray used to sterilize the grafts
- Low doses of radiation retaining more growth factors
- New foil packaging offers a 5-year shelf life
- More growth factors and cytokines to survive the sterilization process
AURORA APPLICATION
AURORA POST-PROCEDURE BEST PRACTICES
Now that you’ve treated your patient with Aurora Amniotic Membrane, it’s important to follow the below post-procedure steps to ensure the best patient outcome.
- After placing Aurora, select a high dK contact lens that is a little flatter than the lens normally used to fit the patient for normal wear. For example, if a corneal curvature suggests the patient requires an 8.6 lens, the resultant curvature with the membrane will be a bit flatter, so you may go with a high dK 8.7 lens.
- Instruct the patient to use non-preserved lubricant drops every 3 to 4 hours while awake. In addition, prescribe either a non-viscous antibiotic drop 2-3 times per day or a combination antibiotic steroid twice a day, depending on which is appropriate for the given case.
- Have a staff member contact the patient the following morning to ask how he/she is doing. If the patient reports mild to moderate blur and a mild foreign body sensation, reassure him/her that this is normal.
- If the patient reports redness, pain, swelling or photophobia, encourage the patient to come into your practice the same day. Usually these symptoms are due to mild hypoxia related to the contact lens becoming dry and/or tight.
- If patient is doing well, schedule an appointment for day 3. At that time, look to see if the membrane is fully broken down. If this is the case, remove the bandage lens and initiate a continuous regimen. If further breakdown of the membrane is needed, send the patient home and schedule another appointment in 2-3 days to reassess.